Volunteer Water Quality Monitoring
Introduction
Regulatory bacterial monitoring is only mandated for public swimming beaches. Concern over the limited scope of this policy, which ignores other shoreline environments, has prompted the development of many community-based water quality monitoring programs. These programs are effective in accurately monitoring the bacterial status of surface waters and these efforts promote education and environmental ethics amongst resource users.
Sources of Bacterial Contamination
Storm water runoff, septic system effluent, run-off from agricultural and recreational areas such as public beaches, trailer parks and marinas, discharge from boats and fecal material from wildlife and pets all represent potential sources of bacterial contamination in surface waters.
Bacterial Indicators and Regulatory Guidelines
Bacterial indicators are enumerated in natural waters to assess the presence and level of fecal contamination and thus determine the potential for disease. Total Coliforms, Fecal Coliforms and Escherichia coli (E. coli) are widely used in regulatory monitoring of recreational waters. However, E. coli is recommended as the main indicator of fecal pollution (WHO, 2000). These indicator organisms themselves are not the cause of illness; however, their presence is used to indicate the risk that other pathogenic organisms of fecal origin may be present.
Total Coliforms are abundant in the feces of all warm-blooded animals, but are also found in the natural aquatic environment, in soil and on vegetation. Unlike the general Coliform group of bacteria, E.coli is exclusively of fecal origin, and it’s presence is thus an effective confirmation of fecal contamination.
Ontario Recreational Guideline for E. coli
If levels of E. coli in surface waters are found to be greater than guideline levels, it is probable that pathogenic organisms are also present. Swimming in bacterially contaminated waters could result in gastro-intestinal symptoms, eye infections, skin complaints, ear, nose and throat infections and respiratory illness.
The most recent Ontario recreational water quality standard is a geometric mean concentration (minimum of five samples) of less than 200 E. coli/100 mL, and a single-sample maximum concentration of less than 400 E. coli/100 mL, while the previous standard was less than 100 E. coli/100 mL as a geometric mean.
Alternative Water Quality Objective
According to the Province of Ontario, surface water is safe for recreational purposes if there are less than 200 E. coli/100 mL (this concentration represents an acceptable threshold of risk), however the presence of this concentration of E. coli in surface waters is indicative of deteriorating water quality. This guideline may be suitable for highly urbanized regions (where water impairment has already occurred) however, a more stringent objective for E. Coli may be more suitable for regions where the water quality has not yet been impaired. For this reason, a Water Quality Objective of 10 cfu’s E. coli/100mL has been used in water monitoring programs in several Ontario lake communities (i.e. Georgian Bay and Muskoka regions).
Monitoring Design
Sampling Sites
Sampling sites should be distributed around the lake (river/waterbody) and should focus on those areas with the greatest potential for water quality impairment. These tend to be areas where sources of contaminants are concentrated such as those with high density development, impermeable/unnatural shorelines, recreational areas, marinas, etc.
Once sampling sites have been determined, make note of the location including landmarks so the sample site location remains consistent. If possible, determine the coordinates with a GPS device/smart phone.
A suitable control site(s) should also be selected. Ideal control sites would have no development or human activity (if this is possible) within the surrounding area. These sites are necessary for providing comparative data and information on background bacterial levels.
All sampling sites should be clearly identified on a map. The sites should remain consistent for future years to provide a comparable and long-term database.
Sampling Schedule
A biweekly (once every two weeks) sampling schedule is often adequate for bacterial monitoring; however, post heavy rainfall event sampling should also be included. Bacterial testing in developed areas or recreational areas (i.e. beaches, marinas, etc.) is most informative after a major rainfall event and gives insight into the impact of storm water runoff in near-shore waters. Include at least two post rainfall events in your annual program, collecting samples within 12 hours of the event. Clearly identify these event-related samples for later interpretation.
In high density resident/cottage and recreational areas such as parks, campsites, beaches and boat anchorage bays and marinas, testing is often most effective immediately following peak usage times, such as Sunday or Monday mornings. Samples should be collected in the morning before 10 a.m. as solar radiation can kill bacteria. For seasonal areas the program should cover the high intensity use period, which is often from June until September.
Sample Collection
Sample Bottles:
250-500mL plastic (i.e. laboratory grade Nalgene sample bottles which can withstand boiling water) or glass sample bottles (or jars) may be used. Each bottle should be labelled with a sampling site number and/or description of the site and always re-used for the same site. Use a waterproof marker or water-resistant label.
Sampling Procedure:
The sample bottle should be rinsed out several times using water at the sampling site prior to collecting the water sample. The water sample should be collected from just below the water surface (around elbow depth) but not including the surface film on the water.
Grip the bottle at the base and plunge it in a downward motion (bottle going into the water top down) into the water to a depth of 9-15 inches.
Sample Storage/Transport:
Water samples should be kept in a cooler with ice packs, at a temperature of 4 to 8° C. Do not allow the sample water to freeze.
Observations and Data Sheet
Complete a data/field sheet for each site including the following information:
- Name of site
- Date
- Time of sampling
- Name of volunteer
- Weather conditions (i.e. sunny, overcast, relative wind strength)
- Recent precipitation
- Air and water temperature
Additional Observations:
Record any other factors or conditions that may influence sample results such as cloudy water, visible waterfowl, or any unusual activity in the area.
Secchi Disk (optional):
Secchi Disk measurements can be taken to measure water clarity and is the standard method for determining water clarity in freshwater lakes. Water clarity provides an indirect measure of nutrient loading into lakes, since low nutrient levels result in less phytoplankton bloom, and therefore greater water clarity. Conversely, higher nutrient levels often result in reduced water clarity. The nutrients of concern are phosphates and nitrates from septic systems, fertilizer runoff and use of soaps and detergents.
Bacterial Analysis with ColiPlate™
Filling the ColiPlate:
- Keep each sample refrigerated until you are about to fill the ColiPlate. Samples should be plated within 12 hours of sample collection.
- Plug in incubator and bring it up to 35° C (95°F) – 37° C (98.6° F). Start this process at least one hour before samples are loaded. Place the thermometer on the bottom of the incubator in a position that can be easily seen through the viewing window (HovaBator Incubator).
- Remove samples from the fridge.
- Match each sample with a ColiPlate by writing the site name or number on the ColiPlate label.
- Wash your hands thoroughly.
- Remove the ColiPlate lid. Shake the water sample vigorously before opening the lid (this distributes the bacteria in the water sample). Gently pour a small stream of sample water onto the plate, running the stream along each row of wells so that water enters each well. When all wells are full and excess sample water remains on the plate, gently tap the side of the ColiPlate to dislodge any air bubbles which may remain in the bottom of some wells. To ensure all wells are full, view the plate at eye level and top up any wells which are not full.
- To remove excess water from the ColiPlate and in between wells, tilt the plate on an angle and drain off. In fact, the ColiPlate can be carefully tilted to a 90° angle to assist the draining of water, while keeping the sample water in each of the 96 wells. Use a paper towel or tissue (using a new one for each plate) to wick away the last few drops of water at the low corner of the plate (and around the plate if needed). Viewing the surface of the plate at eye level should reveal that all wells are full, that the surface water on each well has a concave shape, and that no excess water remains on the surface (or in between the wells) of the plate. If any wells remain unfilled, top up with a little more sample and drain off excess. Proper drainage of water in and in between the wells of the ColiPlate is very important and ensures that cross contamination does not occur.
Tip: If you are having difficulty draining the ColiPlate, so that when replacing the lid on the plate, water is spreading overtop the wells, you can incubate the plates without the lid on. Make sure you place the labeled lid next to the plate (or some other labelling method) so it can be identified after incubation.
ColiPlate Incubation:
- When all ColiPlates are filled place them into the incubator, making sure you can observe the temperature. ColiPlates can be stacked, however be careful not to overload by stacking the plates too deep, this can affect airflow.
- Replace the lid of the incubator and allow the temperature to re-stabilize to 35 – 37° C. This may take close to an hour.
- Note the time when the temperature re-stabilized.
- Incubate for 24 hours from stabilization time
- Inspect the incubator several times to ensure it is remaining at the appropriate temperature and adjust if necessary.
- After 24 hours remove the ColiPlates from the incubator.
NOTE: Do not incubate for longer than 30 hours. The media in the CoiPlate contains a reagent which inhibits the growth of non-Coliform bacteria, which becomes increasingly ineffective after that time, resulting in potential false positives.
ColiPlate Enumeration:
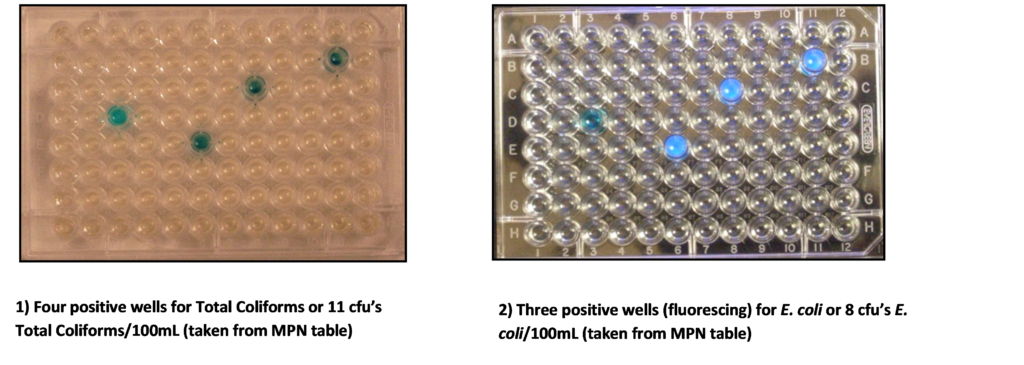
- Place the incubated ColiPlate on a white surface and count the number of wells which turned blue. This is the positive reaction for Total Coliforms. There will be different tones of blue depending on the strength of the bacterial colony in each well. Count ALL blue, blue/green and greenish wells as one count each, regardless of the intensity of the colour change. Refer to the MPN Table to determine the Most Probable Number for Total Coliforms in 100mL of sample water.
- Place the incubated ColiPlate on a black (dark) surface in reduced light and observe under a long wavelength UV light. Count the number of wells that turned blue and were fluorescent under the UV light. This is the positive reaction for E. coli. Do not count wells with fluorescence that are not also blue – they must be blue and fluorescent. Refer to the MPN table to determine the Most Probable Number for E. coli in 100 mL of sample water.
ColiPlate Clean up and Disposal:
- Please rinse out ColiPlates and lids well. Exposed ColiPlates do not contain toxic or pathogenic materials and can be rinsed and drained in a sink. Once they are rinsed and excess water is removed from the wells they can be placed in a closed box and returned for recycling.
- After use, sample bottles and lids should be sterilized by placing them in boiling water for at least 5 minutes and air dried on a clean rack. Seal bottles with lids and set aside for the next sampling date.
Interpretation of ColiPlate Results
Total Coliform and E. coli data (MPN values) for each site should be recorded and interpreted by comparing the individual values to the Provincial Guidelines for Recreational Water (or the more stringent Water Quality Objective for unimpaired lake environments such as Georgian Bay).
If these MPN values remain well below these guidelines, bacteriological water quality is considered to be excellent, and the sampled waters are safe for swimming.
A more site-specific interpretation is the comparison of individual site E. coli counts with other sites in the region, in relation to the difference in the amount of development/human activity between the sites. A useful comparison is the difference between bacterial values found in the control site(s) versus those found in more developed sites. This information gives insight to the impact development/human activity is having on local bacteriological water quality.
Bacterial data, in particular E. coli results for each site should be averaged (geometric mean should be used) for longer term trend analysis purposes. Documentation of changes in bacteriological water quality trends provides valuable information in lake planning and resource management initiatives.
Quality Control
Quality control measures are an important component of all water monitoring programs.
This includes:
Field Blank: use sterilized distilled water to ensure that the sample bottles are being sterilized properly, the bottles are being handled correctly and that the appropriate procedures are being followed to process samples using the ColiPlate technique. Label the bottle as ‘Field Blank’ and follow the same procedures as the other water samples.
Field Duplicate: use the same sample water (use a 500 mL sample bottle) from a pre-selected site and fill two ColiPlates (one for the site and one for the field duplicate). Record and compare the results of the duplicate sample. While the results should be similar, bacteria are not evenly distributed in water (even after shaking the sample), and tends to clump together or onto particles, so there may be a small difference in counts. Remaining sample water can also be refrigerated and transported within 24 hours to an accredited laboratory for Total Coliform and E. coli analysis.
Laboratory analysis: use an accredited laboratory to process the same sample used for the field duplicate and compare Total Coliform and E. coli results.
